Epilepsy is a complex chronic group of neurological disorders that affects about 60 million people worldwide with 6 million in Europe alone. This research plans to investigate the role of the eCBs in human absence epilepsy.
Epilepsy is characterized by spontaneous and recurrent unprovoked seizures (bursts of neuronal hyperactivity) arising in the brain that can be “focal” or “partial” if they remain confined to their area of origin, or “generalized” if they spread to the entire cerebral hemispheres.
Seizures have recently been classified in focal and generalized convulsive and non-convulsive epilepsies according to their different characteristics. Epilepsy can be symptomatic, for example, due to stroke, infections, brain tumors, prolonged febrile seizures, and other occurrences of status epilepticus (SE).
There is currently no cure or prevention for epilepsy. Most if not all of the approved antiepileptic drugs (AEDs) are not truly “antiepileptic” but merely “anti-seizures”. Indeed, the AEDs do not stop epileptogenesis; the process of converting a normal brain to a brain with epilepsy, at the most they reach complete seizure control. Unfortunately, not all PWE respond to the therapies with 30-40% of them having pharmacoresistant epilepsy. Moreover, PWE with longer duration of active epilepsy show higher comorbidity of depressive disorders, bipolar disorder and anxiety.
Although the efforts in antiepileptic drug development have not resolved this issue, they have encouraged experimental and clinical research to focus on different mechanisms involved in the neurological disorder. Indeed, many candidate processes and molecular targets are currently under intense scrutiny and hopefully will improve treatment and quality of life of PWE.

Treatment for epilepsy based on Marijuana preparations, containing plant cannabinoids such D9-THC, cannabidiol (CBD) and cannabinol (CBN) (known as phytocannabinoids), has received prominent attention in the lay press and in social media, and recently also in the scientific community, with reports showing dramatic improvements in seizure control in children with severe epilepsy.
Notwithstanding this anecdotal evidence of anti-epileptic effects of cannabinoid receptor (CBR) activation, evidence to the contrary is also available. More compelling animal evidence shows that CBR ligands have preferential antiepileptic effects depending on dose and model used.
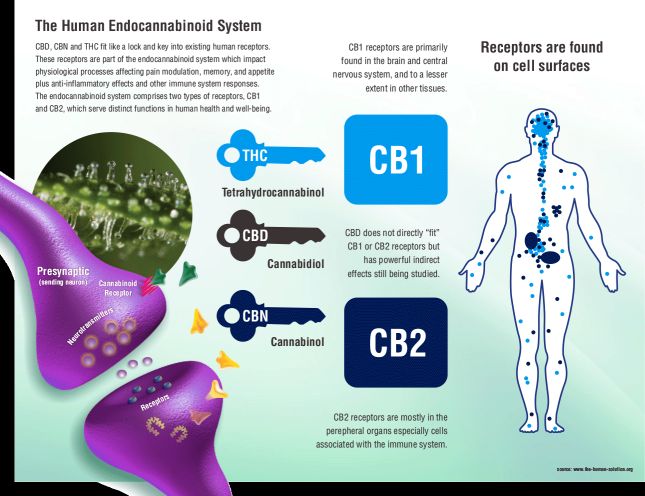
The active ingredient in marijuana, D9-THC, is active in humans because our brain produces its own marijuana compounds that are called endogenous cannabinoids or endocannabinoids (eCBs). The eCB System system is made up of nerves and receptors which communicate with each other using endogenous cannabinoids produced by the body.
This system of nerve and receptors is larger than all the other different systems of receptors put together, making it the most important system of neurotransmitters in the body. It is also responsible for controlling all of the other systems and maintaining homeostasis or balance.
In 1992, Raphael Mechoulam (Hebrew University, Israel) discovered the first endocannabinoid anandamide (n-arachidonoylethanolamine or AEA) naming it after the Sanskrit word ‘ananda’ (bliss) and ‘mide’ (chemical). In 1997, Daniele Piomelli (University of California, USA) discovered lipid 2-arachidonoyl glycerol (2-AG), the other major endocannabinoid in humans. These neurotransmitters pass on signals in our brains and bind to two cannabinoids CB1 and CB2 receptors.
Two types of cannabinoid receptors have been identified; cannabinoid CB1 receptors are located mostly in the central nervous system, but are also expressed by peripheral neurones, and CB2 receptors are predominantly confined to the periphery. Cannabinoid CB1 receptors are located at many of the sites associated with epilepsy in the brain.
D9-THC binds to these CB1/CB2 receptors to trigger the same mechanisms in the brain as ECBs, and due to the high concentration (much higher than the eCBs) that reaches the brain after a joint for example, it produces the typical effects of Marijuana acute intoxication.
Unfortunately, despite this promising evidence, the current drug regulatory climate, which continues to categorize even non-psychotropic cannabinoids as equivalent to the most dangerous “narcotics”, has made it very difficult to move cannabinoid research forward and toward the development of new cannabinoid-based therapy for the treatment of epilepsy. For medical use, trying to boost our own marijuana (endocannabinoids) would probably be safer. Indeed, we have recently shown that increasing eCB levels, by blocking fatty acid amide hydrolase (FAAH)-mediated AEA degradation with systemic administration of URB-597, reduces seizures in a model of Temporal Lobe Epilepsy, the Maximal Dentate Activation (MDA), without disrupting the hippocampal long term potentiation (LTP), the electrophysiological phenomena underling learning.
No evidence instead exists of a role for the CB system in human absence epilepsy. Moreover, very few studies have investigated the involvement of the CB system in ASs, and their results are highly contrasting. This research project, as stated previously, is to investigate the role of the eCBs in this type of epilepsy.
Typical absence seizures (ASs) of idiopathic generalised epilepsies consist of sudden, brief periods of loss of consciousness which are accompanied by synchronous, generalized spike and wave discharges (SWDs) in the EEG. Similar ASs are exhibited by diverse genetic rat and mouse models, including the Genetic Absence Epilepsy Rats from Strasbourg (GAERS) and Wistar Albino Glaxo/Rij (WAG/Rij) rats. SWDs originate from abnormal firing in thalamic and cortical networks and GABAA inhibition is integral to their appearance. Moreover, an important AS modulation occurs via the basal ganglia, and changes in the firing of GABAergic nigral neurons modulate ASs both indirectly via the nigra/superior culliculus/thalamic projection and directly via the nigro-thalamic pathway.
Despite the progress in advancing our understanding of the neuropathological mechanisms underlying AS, there are still many unanswered questions including the mechanism of action of the gold-standard antiabsence drugs, such as ethosuximide (ETX), valproate and lamotrigine. Moreover, the failure of monotherapy with anti-absence drugs, in >50% of AS patients necessitates the identification of novel therapeutic targets.
Research is needed to develop these new drugs to treat a host of different brain disorders. Several research groups in the world, including us in Malta, are now seeking the answers.
Prof Giuseppe di Giovanni is the lead researcher of this project, which is recipient of the RIDT grant for Brain Research Projects.